For each indication, there is interest in simple, safe and cost-efficient biomarkers which reliably model pathology or can be used supportively in diagnostics. Typical neurophysiological monitoring methods with varying spatial and temporal resolutions are MRI, CT, PET or EEG. EEG components have great potential as biomarkers as they offer a very good temporal resolution and are non-invasive.
On the occasion of the Parkinson Awareness Month, we want to introduce electrophysiological markers for Parkinson’s disease. In the case of PD, the event-related potentials (ERP) P300 and the mismatch negativity (MMN) have received scientific interest. Both reflect early sensory information processes, which are known to be affected by PD (Hansch et al., 1982).
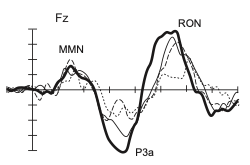
The P300 component is usually captured during so-called Oddball-Experiments in which a deviant stimulus between frequent tones robustly elicits a positive wave of 300 ms after the stimulus onset. However, not only is the performance in PD patients lower but Solís-Vivanco et al. (2015) moreover indicate that the fronto-central P300a is significantly reduced during an acoustic Oddball task in Parkinson patients. The attenuated P300a amplitude may be interpreted as reduced distractibility in PD (Seer et al., 2016). Additionally, the amplitude reduction is influenced by severity and duration of the illness.
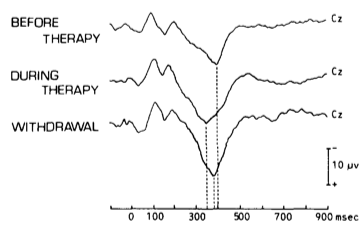
The observed association between illness duration and P3a reduction remains significant even after controlling for demographic variables, indicating potential as a biomarker. Moreover, the P3 shows sensitivity to therapy: The P3 latency is extended in PD patients compared to healthy controls prior to dopaminergic therapy and is reduced during therapy (Stanzione et al., 1991). Although promising results were found in some studies, the applicability of P3 as a biomarker is currently limited by variability in study results. A reduced P3a amplitude as reported by Solis-Vivanco et al. (2015) was only supported partially. According to a recent investigation the differences in the literature are not due to task effects. It rather seems to be a medium-sized effect that does not reach significance in all cases (Seer, Lange, Georgiev, Jahanshahi & Kopp, 2016).
Another ERP component which was investigated was the MMN. The previously mentioned acoustic Oddball task by Solís-Vivanco et al. (2015) requires the participants to respond to one stimulus dimension (e.g. duration of tones), while another stimulus dimension (e.g. pitch of the tone) is varied to allow for frequent and deviant tones. The deviant tone that elicits the P300 ERP also elicits the preceding MMN (see Figure 1.). The Mismatch Negativity (MMN) is evident when changes in auditory stimulation occur and seems therefore to reflect an automatic response to auditory income that differs from the sensory memory trace of preceding stimuli (Seer et al., 2016). However, Solís-Vivanco et al. failed to find a significant difference between healthy and PD patients. By now, the role of the MMN in the context of PD has been studied, but there is no consensus emerging. The evidence is slightly suggesting that an attenuated MMN amplitude may be related to dementia in PD (Seer et al., 2016).
Beyond the neurophysiological markers described here, speech also offers a low-cost biomarker, which puts low burden on the patient. It has been shown that PD manifests in speech and acoustic abnormalities (Polat & Nour, 2020; Goberman, 2005; Karan, Sahu & Mahto, 2020; Gomez-Vilda, 2017). Goberman (2005) demonstrated that speech is related to different symptom clusters of PD (e.g., axial motor symptoms and non-axial motor symptoms).