Using speech to measure cognitive changes in clinical trials and research studies
A 10-minute phone call measures cognitive impairment automatically and remotely
Remotely or in-clinic
SB-C can be used at the patient’s home or in-person at any healthcare facility.
Fully automated or semi-automated
SB-C offers an automated bot option and a semi-automated assisted phone call option.
Over the phone or via App
SB-C integrates 3 user facing frontends: automated phone call, assisted phone call and app.
Fully integrated into third party system or standalone
SB-C can be used out-of-the-box using our Mili system or be integrated into third party systems.
V3 guided, clinically proven
Validation based on large cohorts across Europe following approved methodology from the V3 framework of the Digital Medicine Society (DiME)
- Distinction of clinical groups
- Analytical validation evidence
- Clinical validation evidence
Clear-cut differentiation among healthy controls, MCI and Dementia patients.
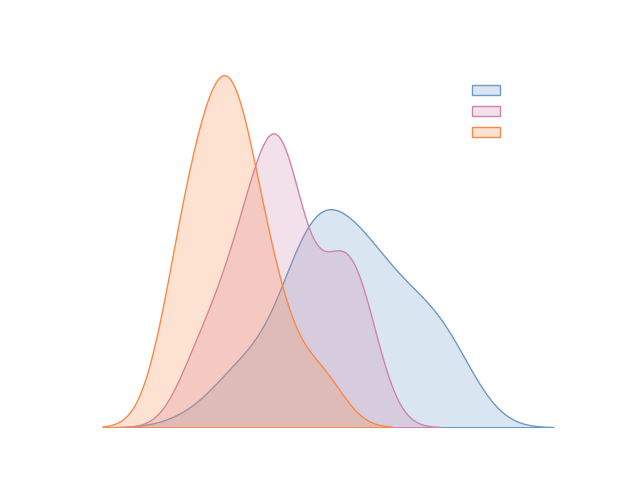
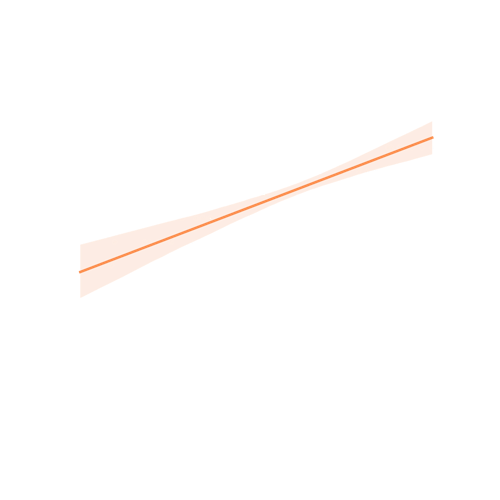
High correlation with clinical cognition rating anchors.
Strong links with AD cerebrospinal fluid markers, such as aBeta and pTau
Previous slide
Next slide
Check out our new validation results on remote speech biomarker for cognition (SB-C) in early Alzheimer’s disease
Tröger J, Baykara E, Zhao J, ter Huurne D, Possemis N, Mallick E, Schäfer S, Schwed L, Mina M, Linz N, Ramakers I, Ritchie C: Validation of the Remote Automated ki:e Speech Biomarker for Cognition in Mild Cognitive Impairment: Verification and Validation following DiME V3 Framework. Digit Biomark 2022;6:107-116. doi: 10.1159/000526471
Cutting-edge technology for real-world solutions
The biomarker can be deployed in any study e.g. using ki:elements mili platform
Highly scalable
Easy process, effortless integration, screening and monitoring with scalability
Cost-effective
Low cost, high quality tool for clinical use
No installation and no smart device
Classical landline or smartphone call to patients
Non-invasive
Non-invasive solution in comparison to blood or CSF biomarkers
7 languages and growing
SB-C is available in (1) English (including US-English, British English and Canadian English), (2) German, (3) Spanish, (4) Swedish, (5) Dutch, (6) French and (7) Catalan
A patient's view on the remote assessment
Usability study for automated and semi-automated testing over the phone
"I did not find it strange doing the questions, conversation via phone."
"... the phone appointment is more convenient than travelling face to face."
Previous
Next
How comfortable do you feel about completing the tasks at home?
🙁
🙂
How comfortable do you feel about completing the tasks on the phone?
🙁
🙂
How easy do you find it to set up the phone appointment?
🙁
🙂
How was the sound quality?
🙁
🙂
Ongoing collaborations on SB-C
We work with a number of KOLs
AuTonomy | J&J
n~2000 – Prodromal AD/MCI
n~2000 – Prodromal AD/MCI
2022
0
PROSPECT AD – EPAD Scotland | Prof. Craig Ritchie
n~300 – Pre-clinical AD
n~300 – Pre-clinical AD
2022
0
PROSPECT AD – ADetect | Prof. Oskar Hansson
n~250 – Prodromal AD/MCI
n~250 – Prodromal AD/MCI
2022
0
PROSPECT AD – DESCRIBE | Prof. Stefan Teipel
n~300 – Pre-clinical AD
n~300 – Pre-clinical AD
2022
0
PROSPECT AD – Beta AARC | Prof. Juan Gispert
n~300 – Pre-clinical AD
n~300 – Pre-clinical AD
2022
0
DAC-SP – COGSCREEN | Prof. Robert Perneczky
n~250 – All-comer
n~250 – All-comer
2022
0
Get in touch
& work with us
Reach out to us via LinkedIn in by clicking on our profiles above or write us an email using the contact form.
We’re looking forward to working with you!